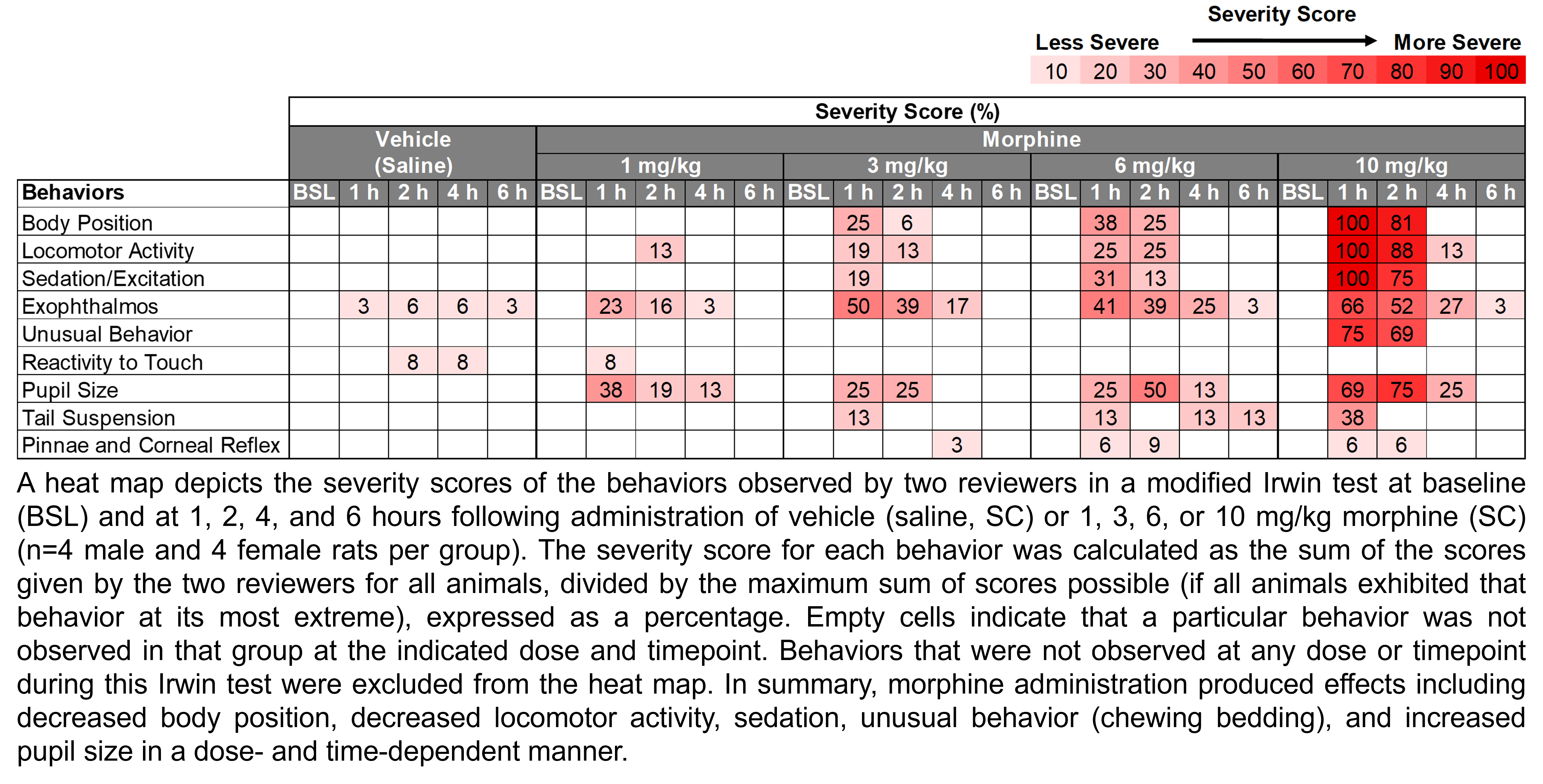
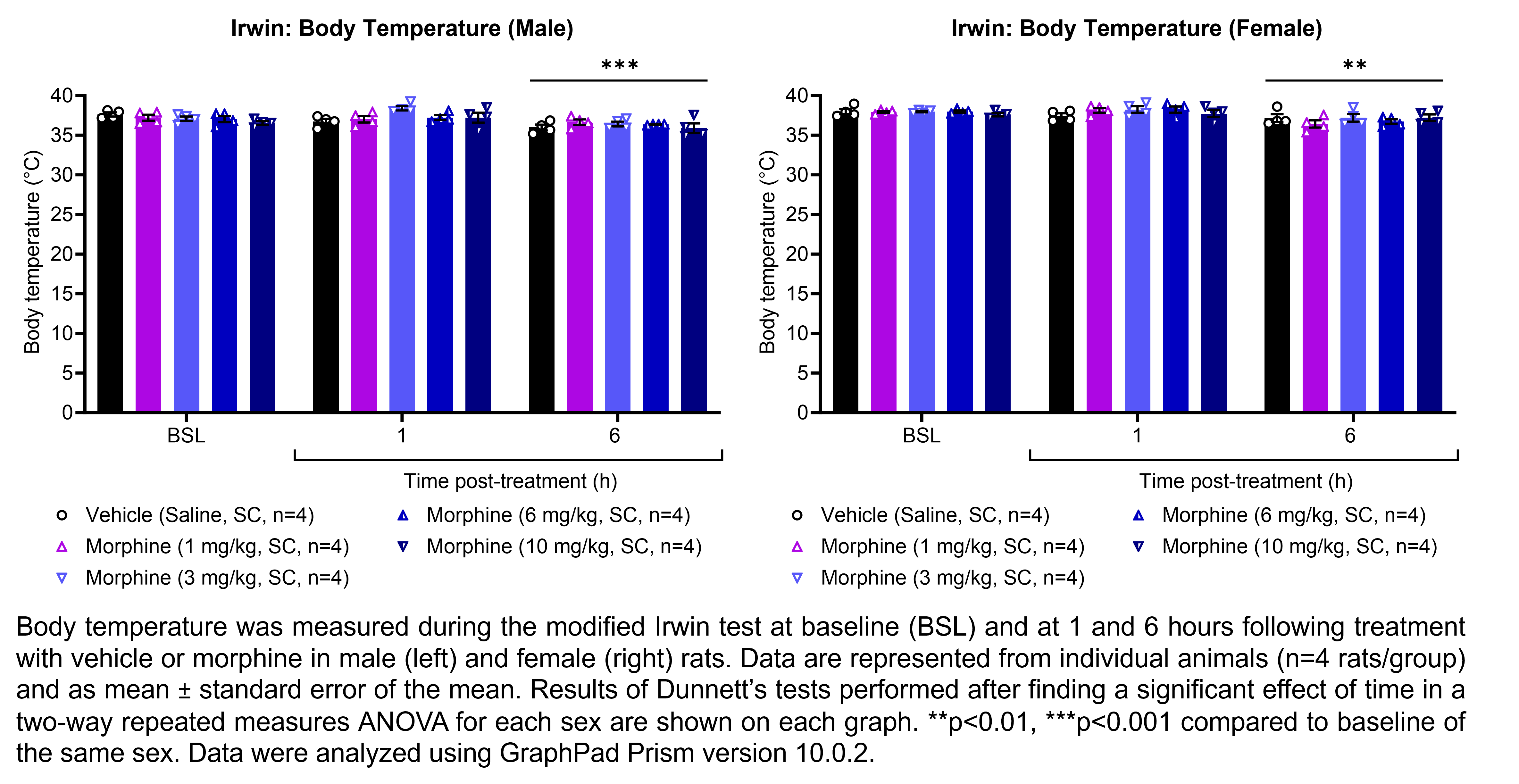
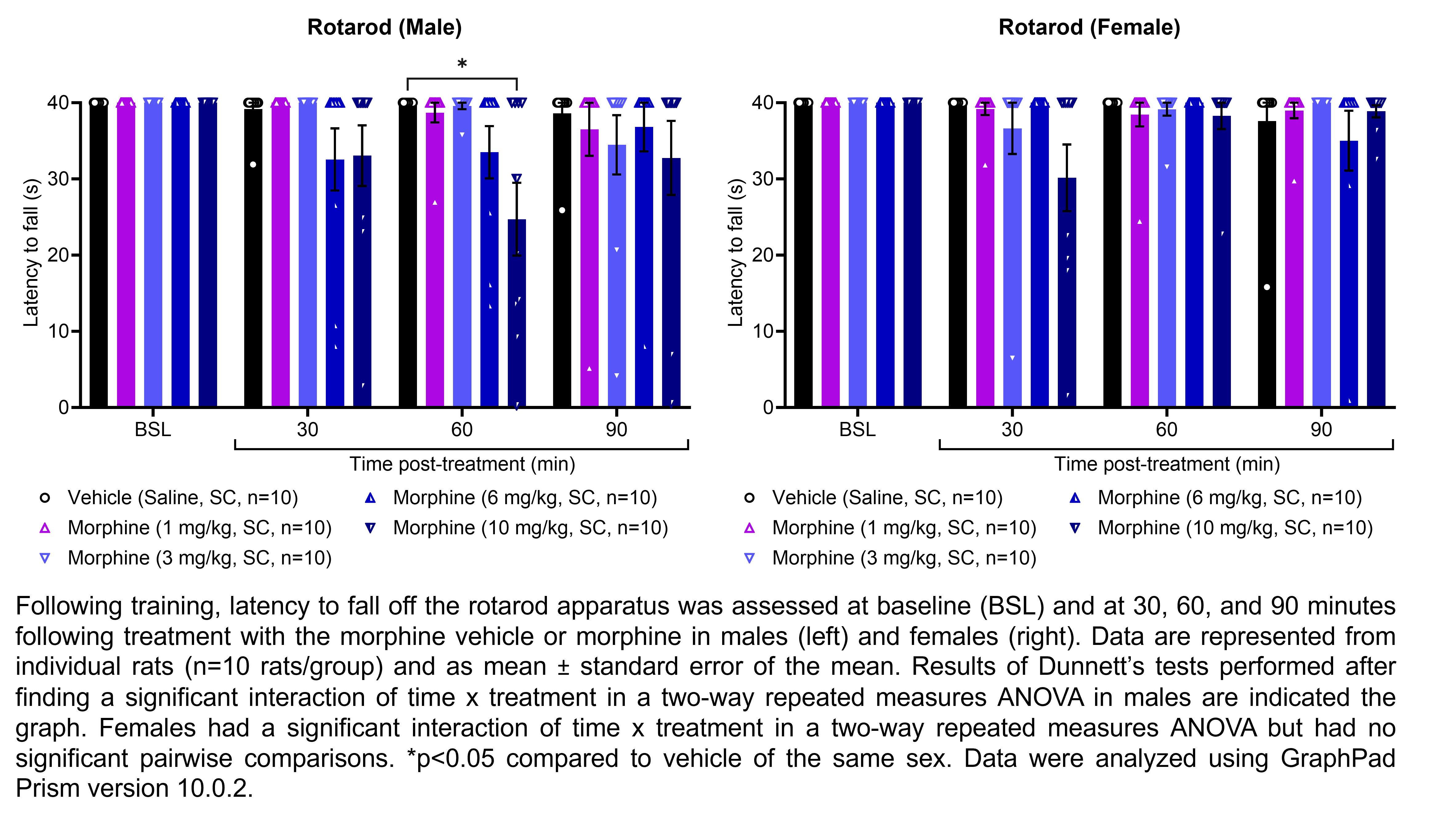
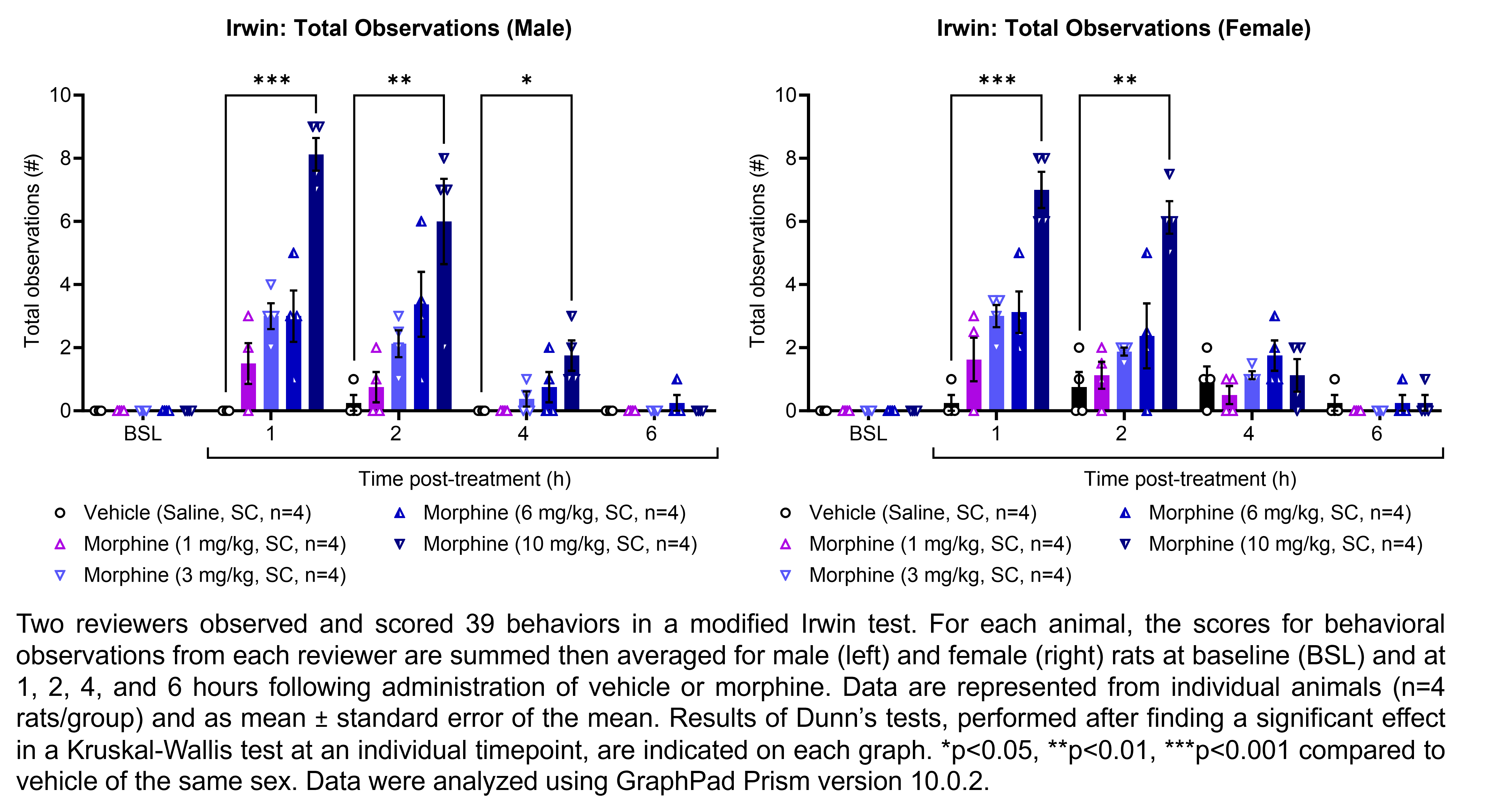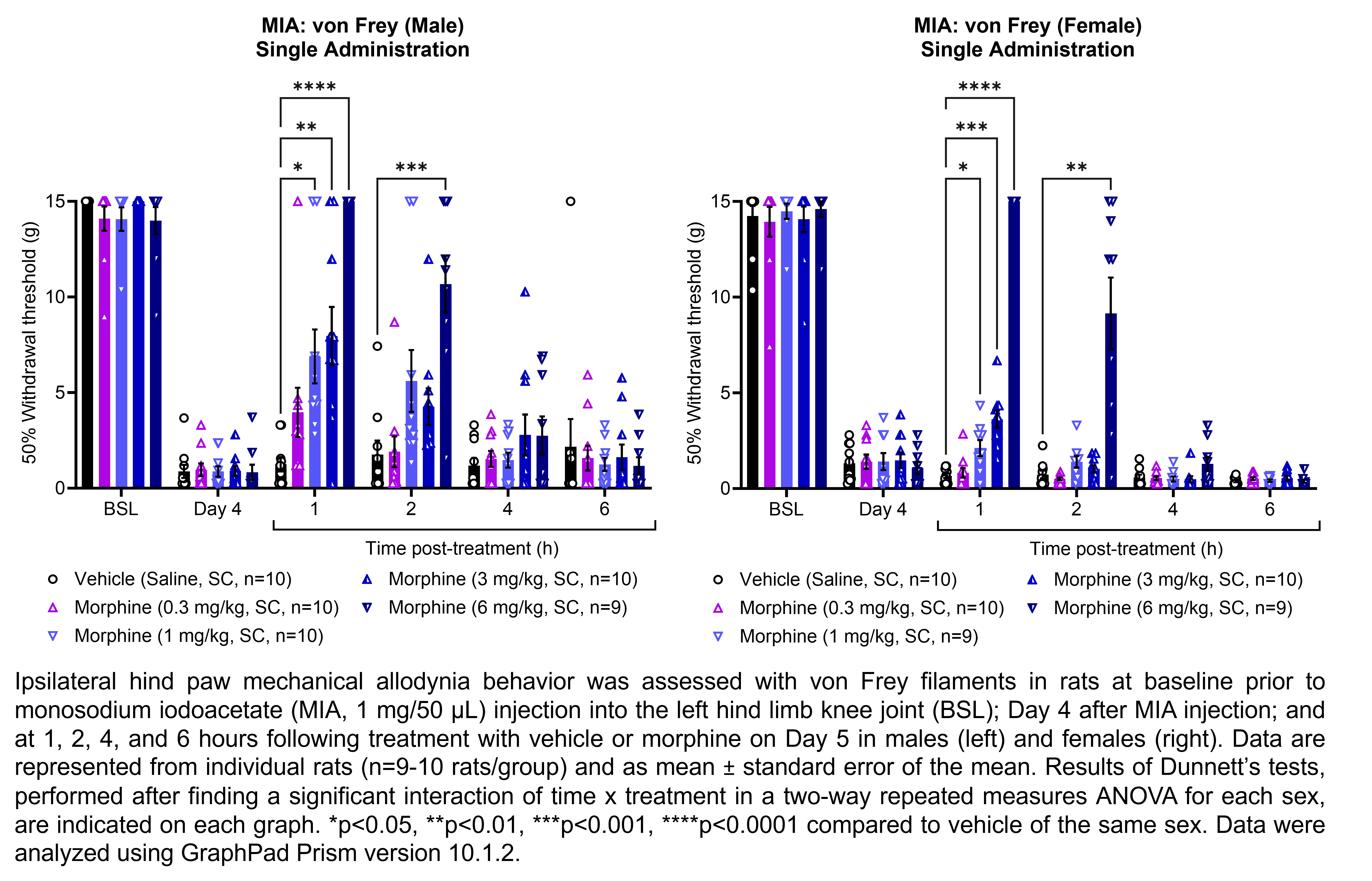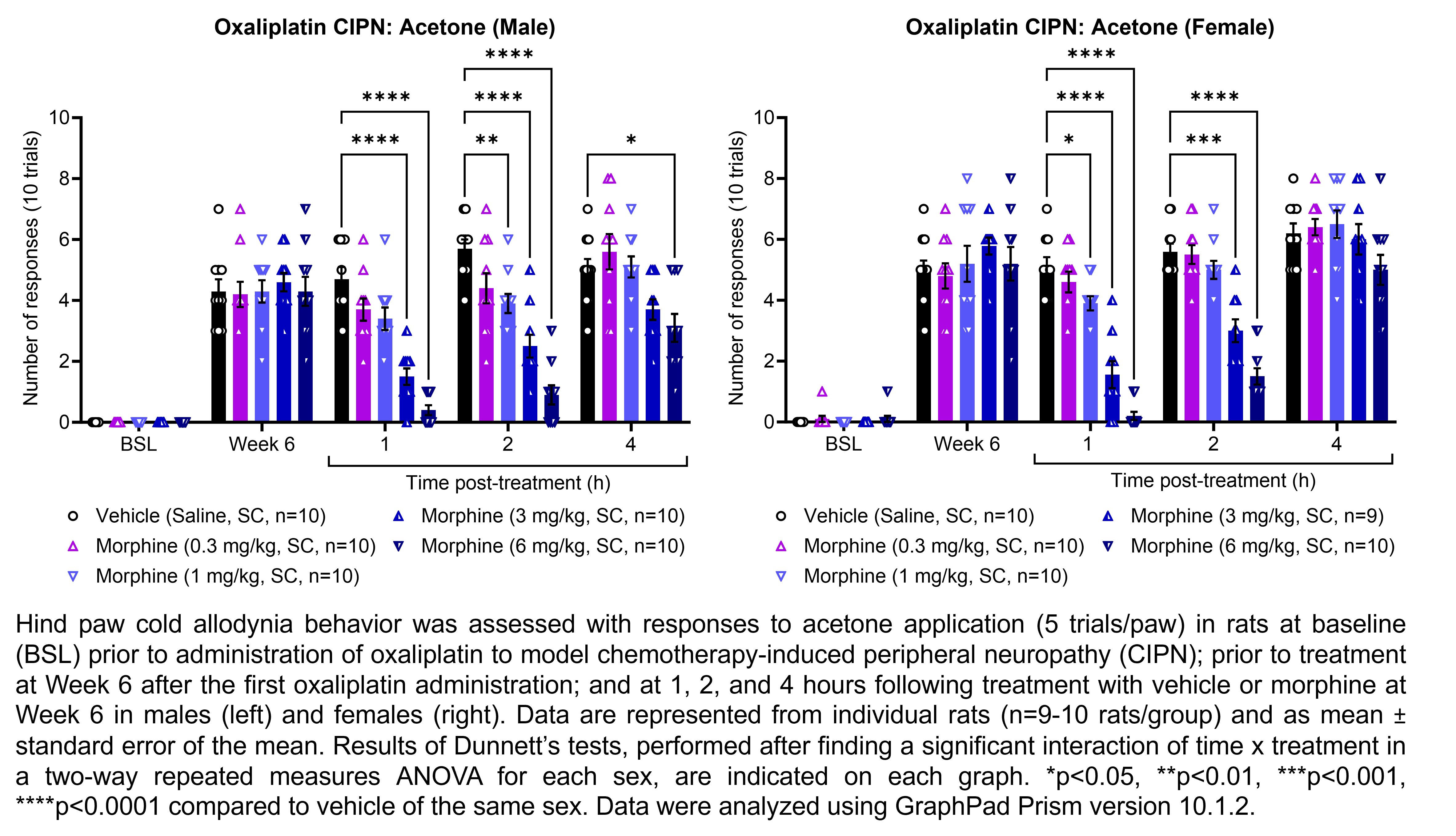MORPHINE
Formulation
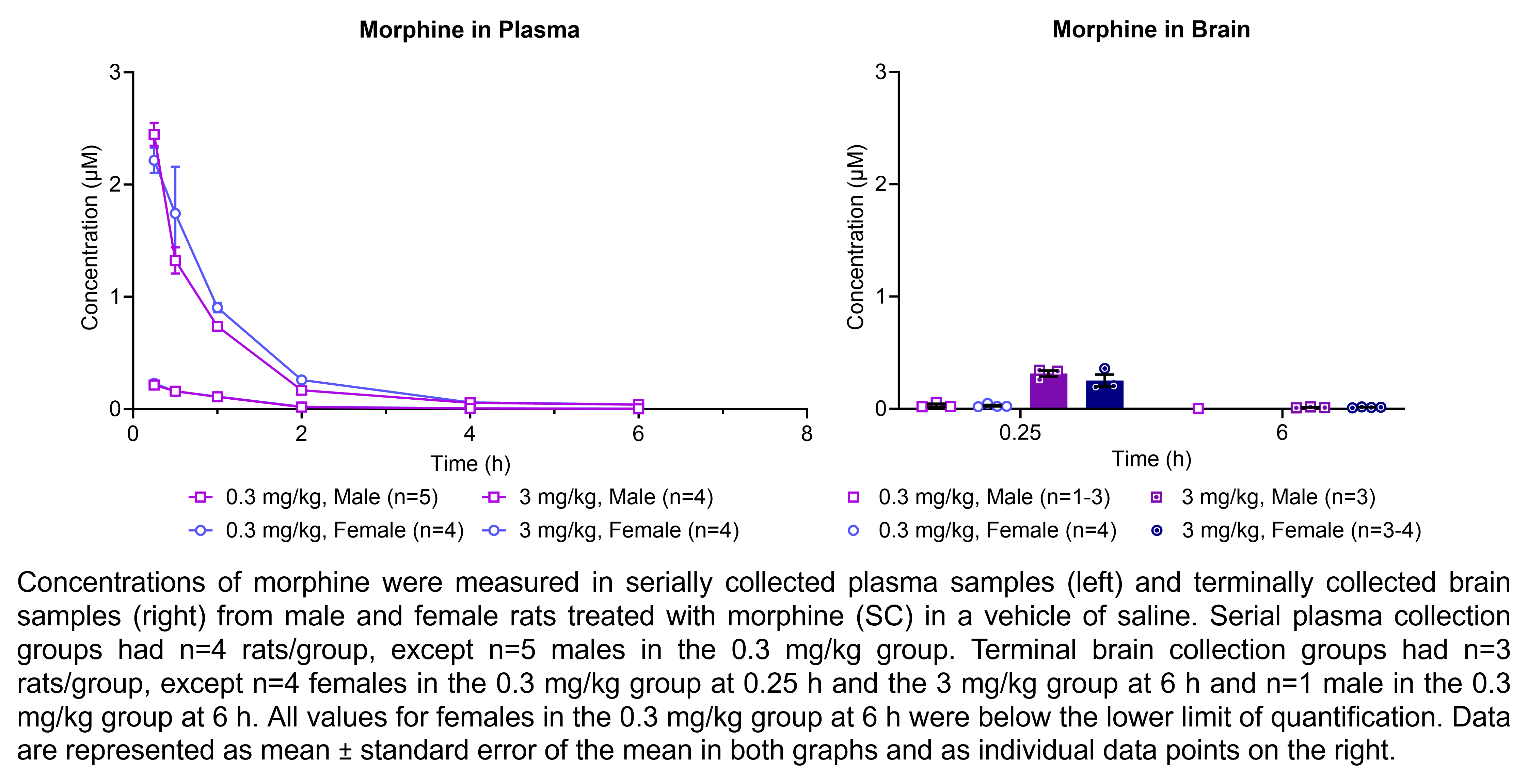
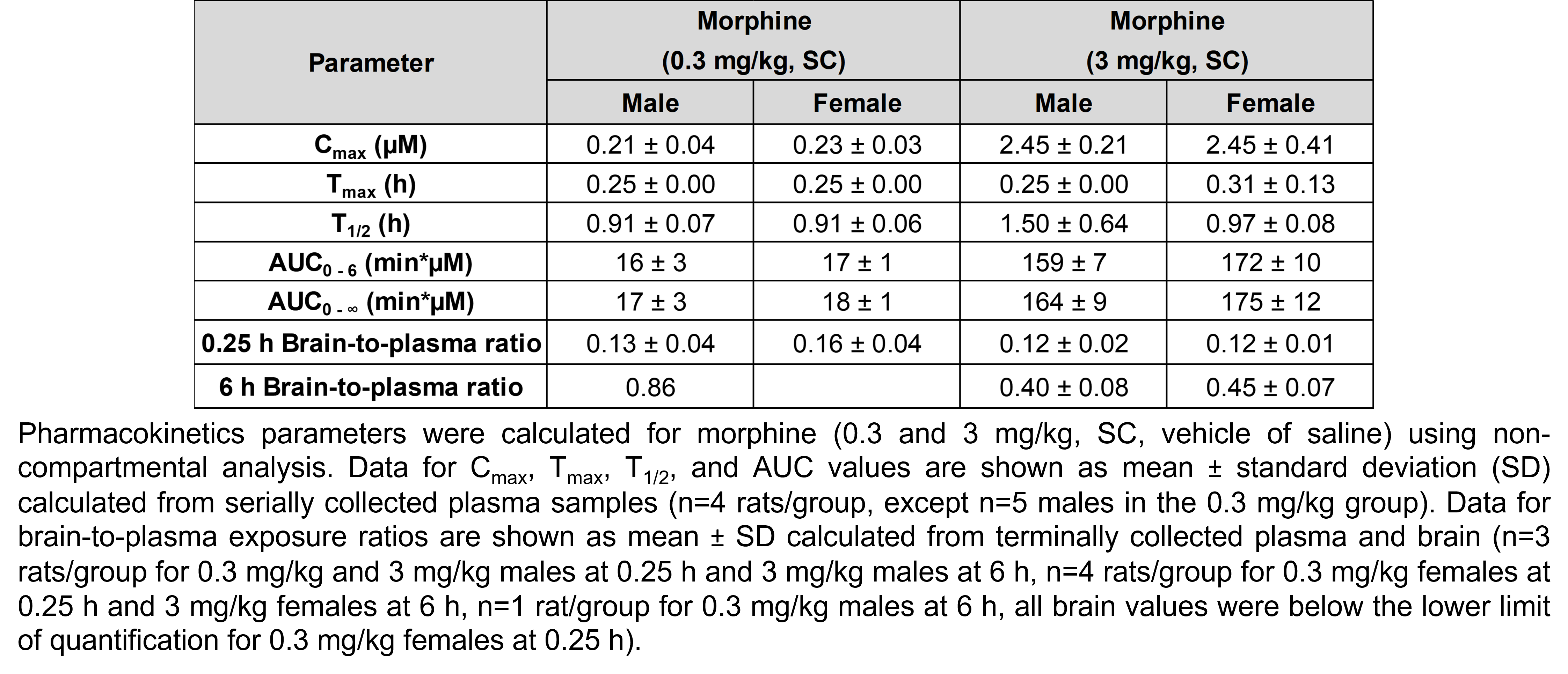
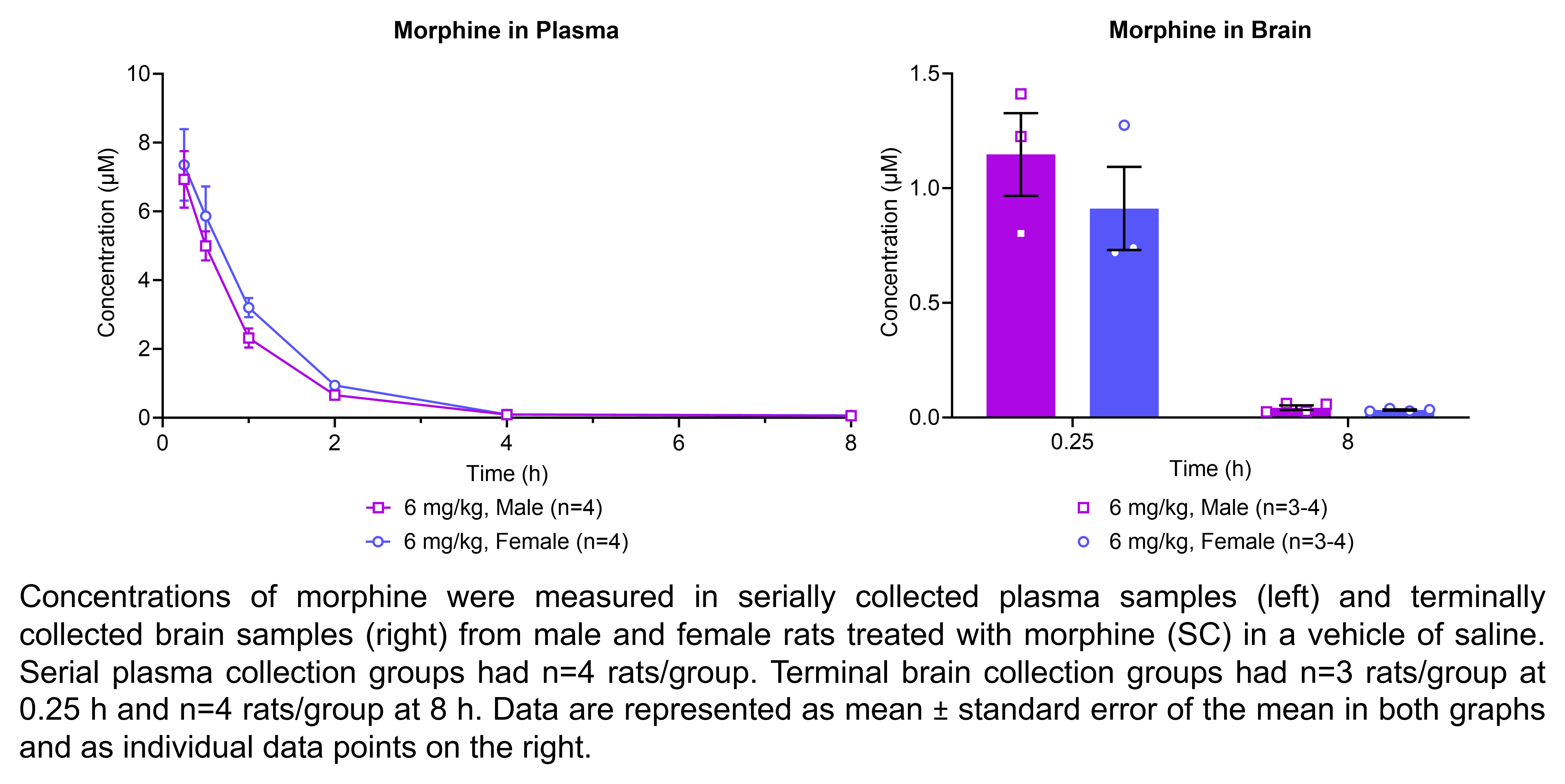
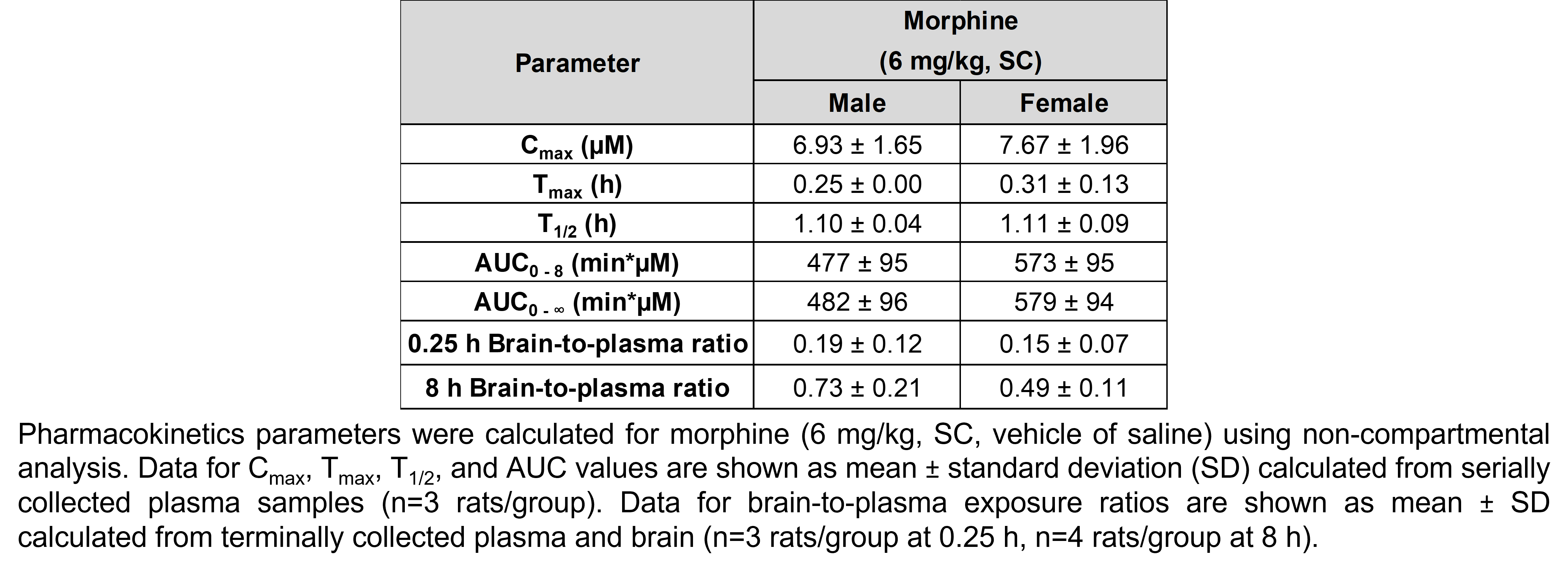
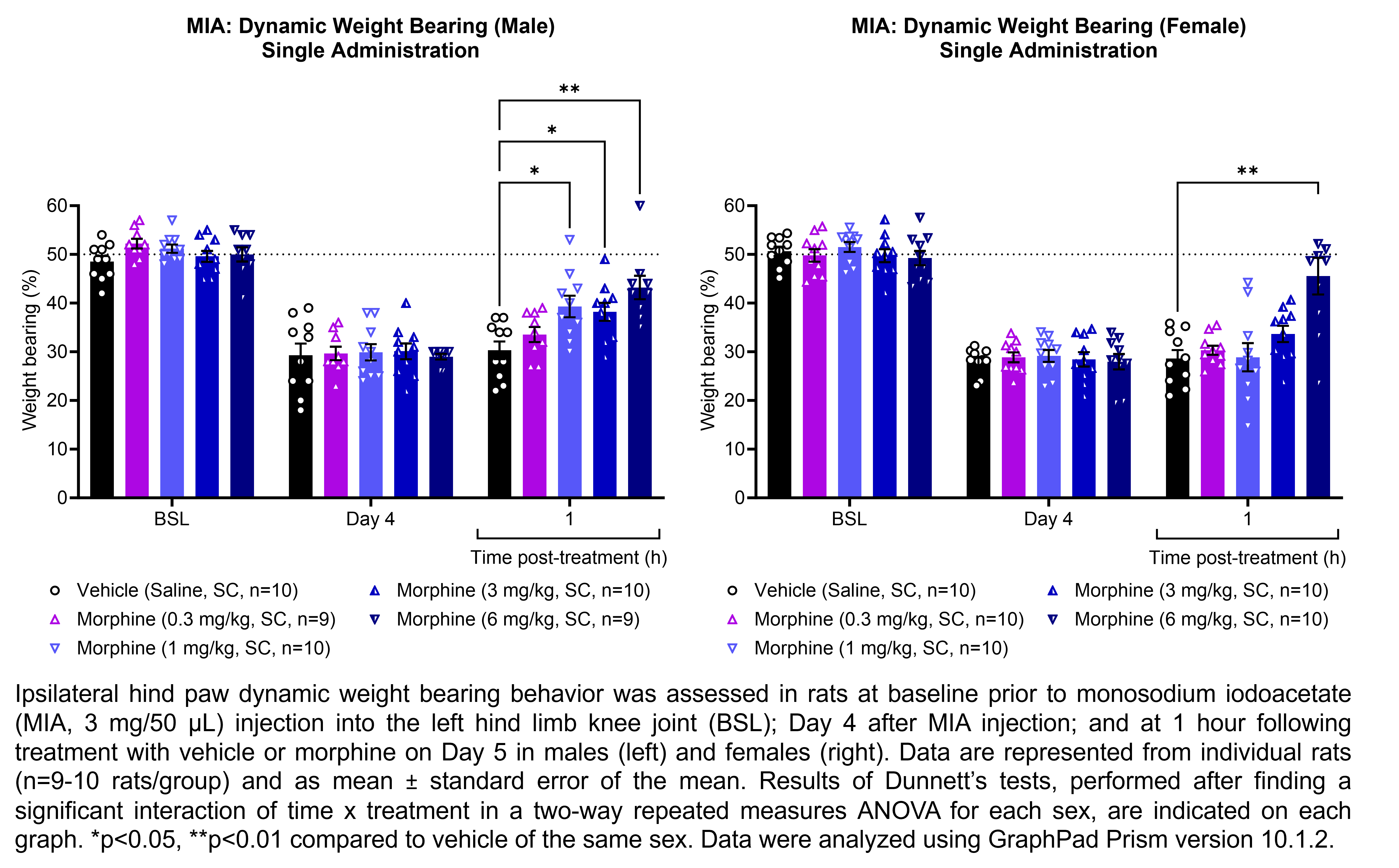
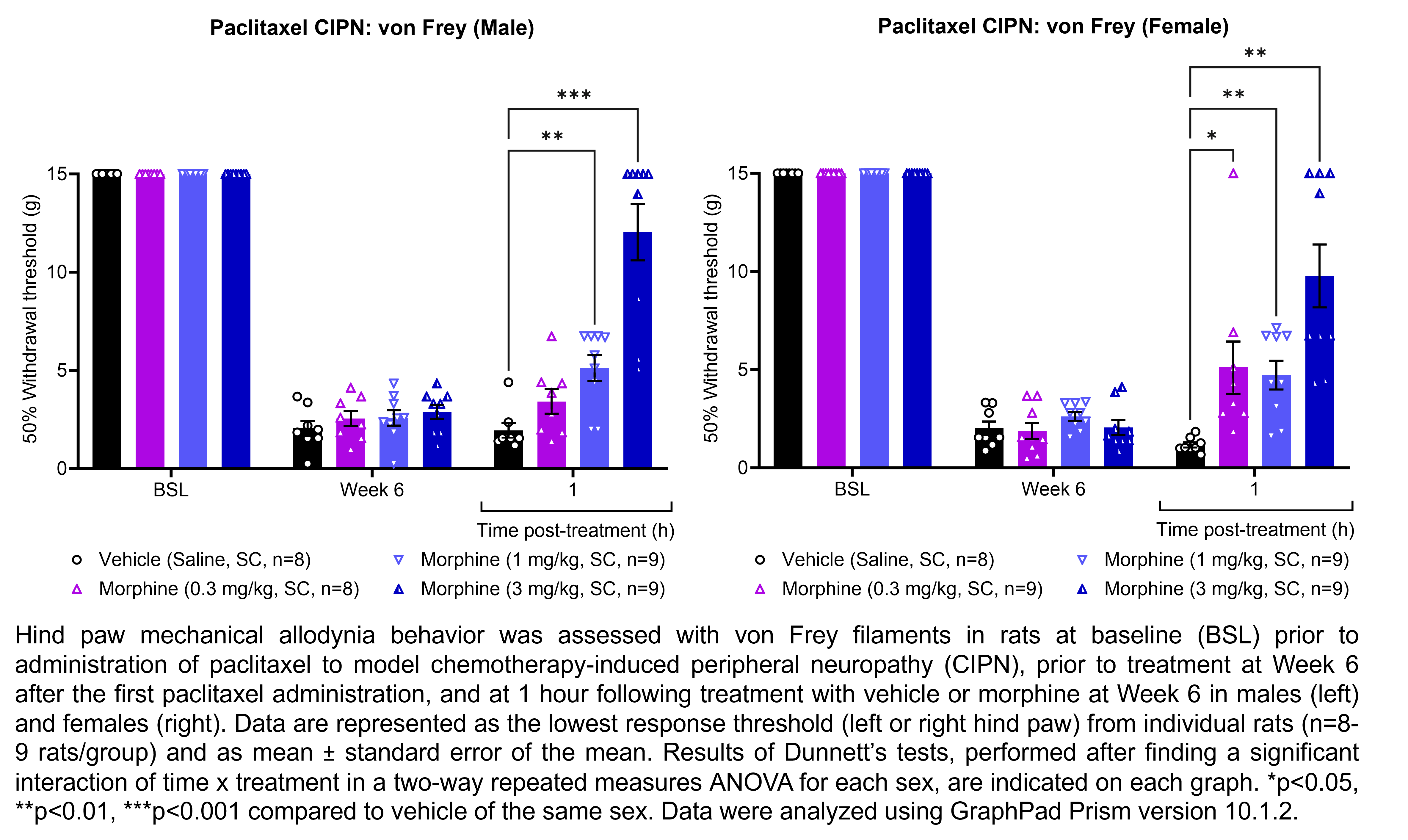
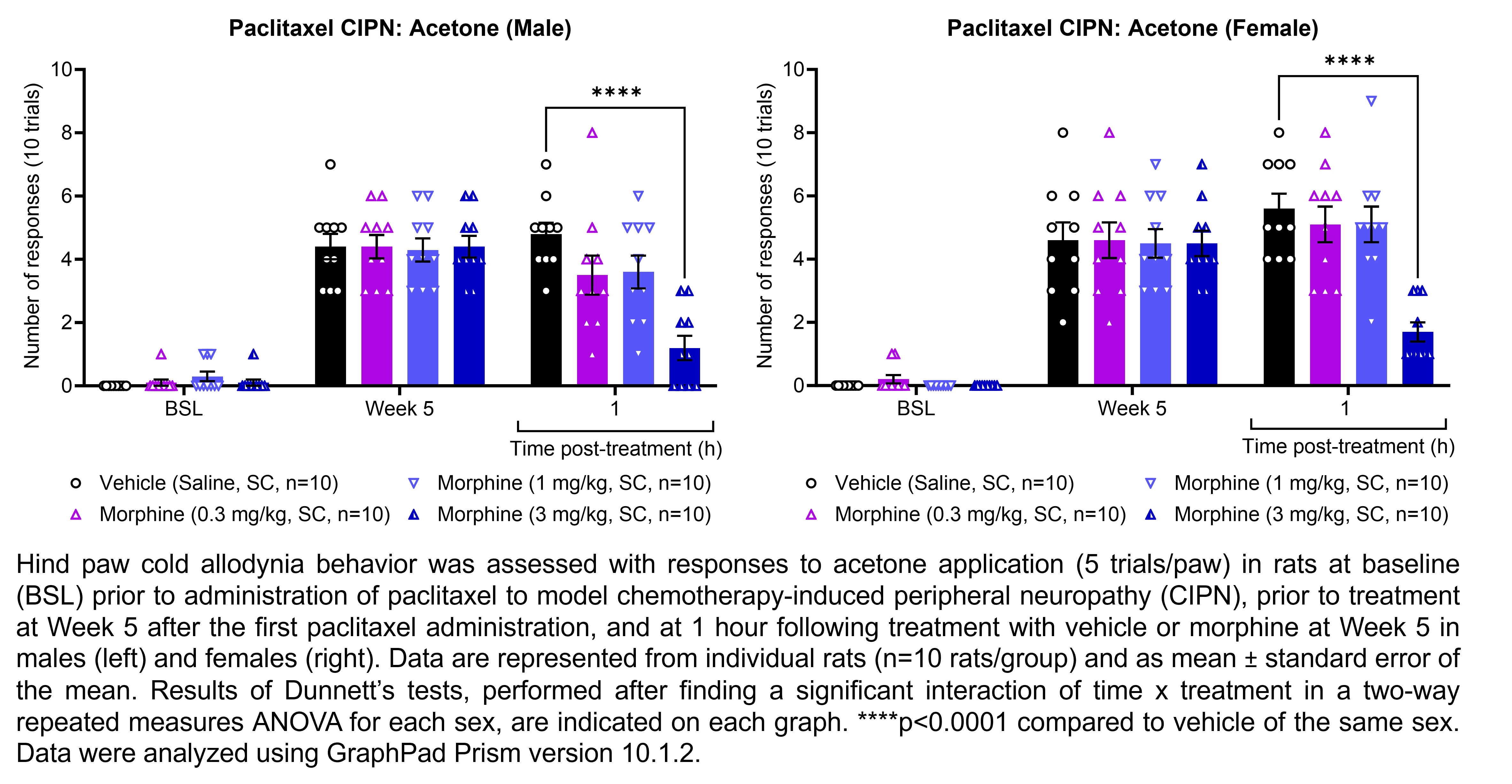
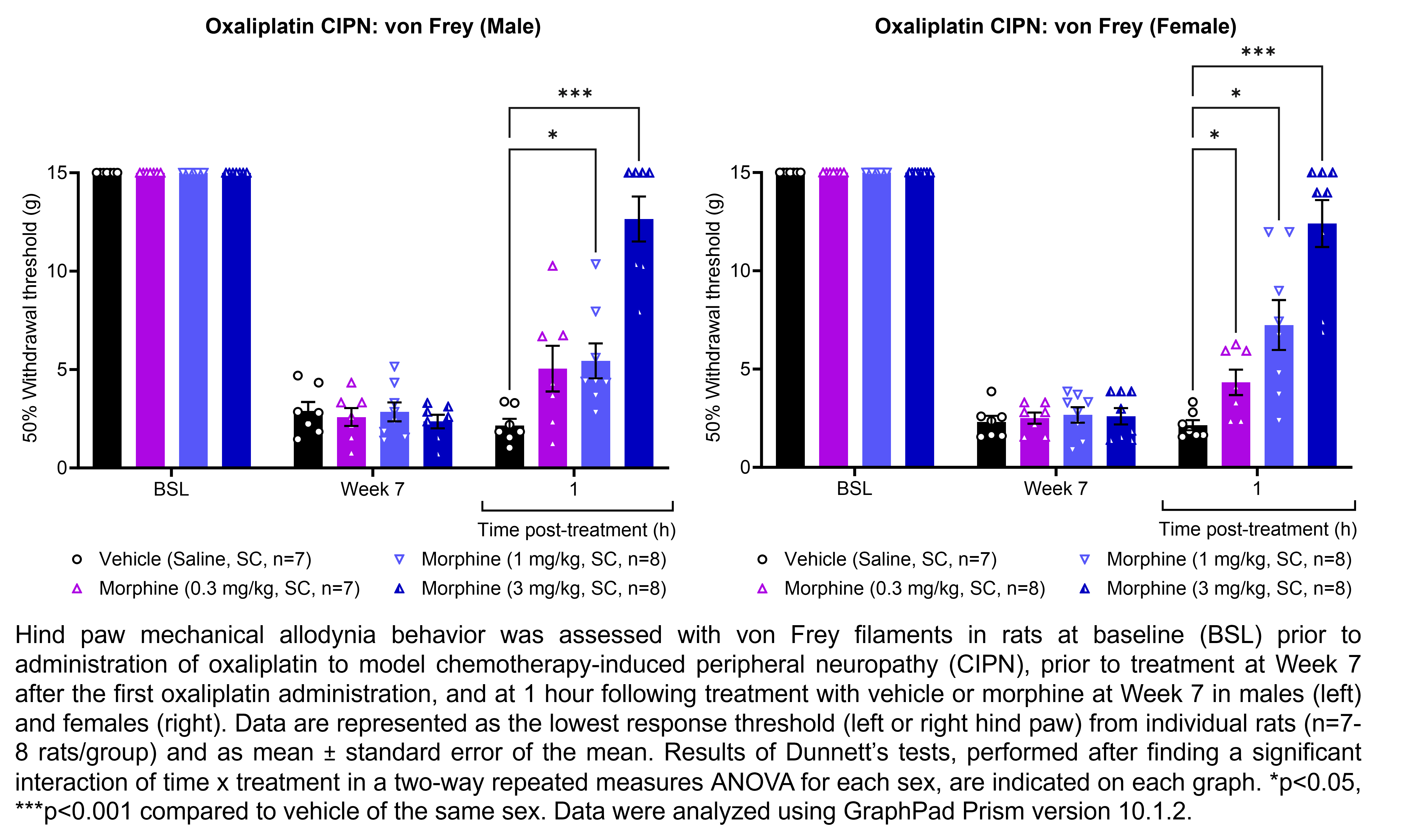
The table below describes the formulation of morphine sulfate and the controls pregabalin, ketoprofen, and gabapentin for the following in vivo experiments. For MIA, paclitaxel CIPN, and oxaliplatin CIPN studies, morphine showed efficacy in all endpoints and is used as the positive control when testing other compounds in these models; therefore, no additional positive control is used in the MIA, paclitaxel CIPN, and oxaliplatin CIPN morphine dose response studies.
| Experiment(s) | Compound | Dose(s) mg/kg | Correction factor | Dose volume mL/kg | Route | Vehicle | Suspension / Solution |
|---|---|---|---|---|---|---|---|
| Pharmacokinetics | Morphine | 0.3, 3, 6 | 1.33 | 1 | SC | Saline | Solution |
| Irwin, Rotarod | Morphine | 1, 3, 6, 10 | 1.33 | 1 | SC | Saline | Solution |
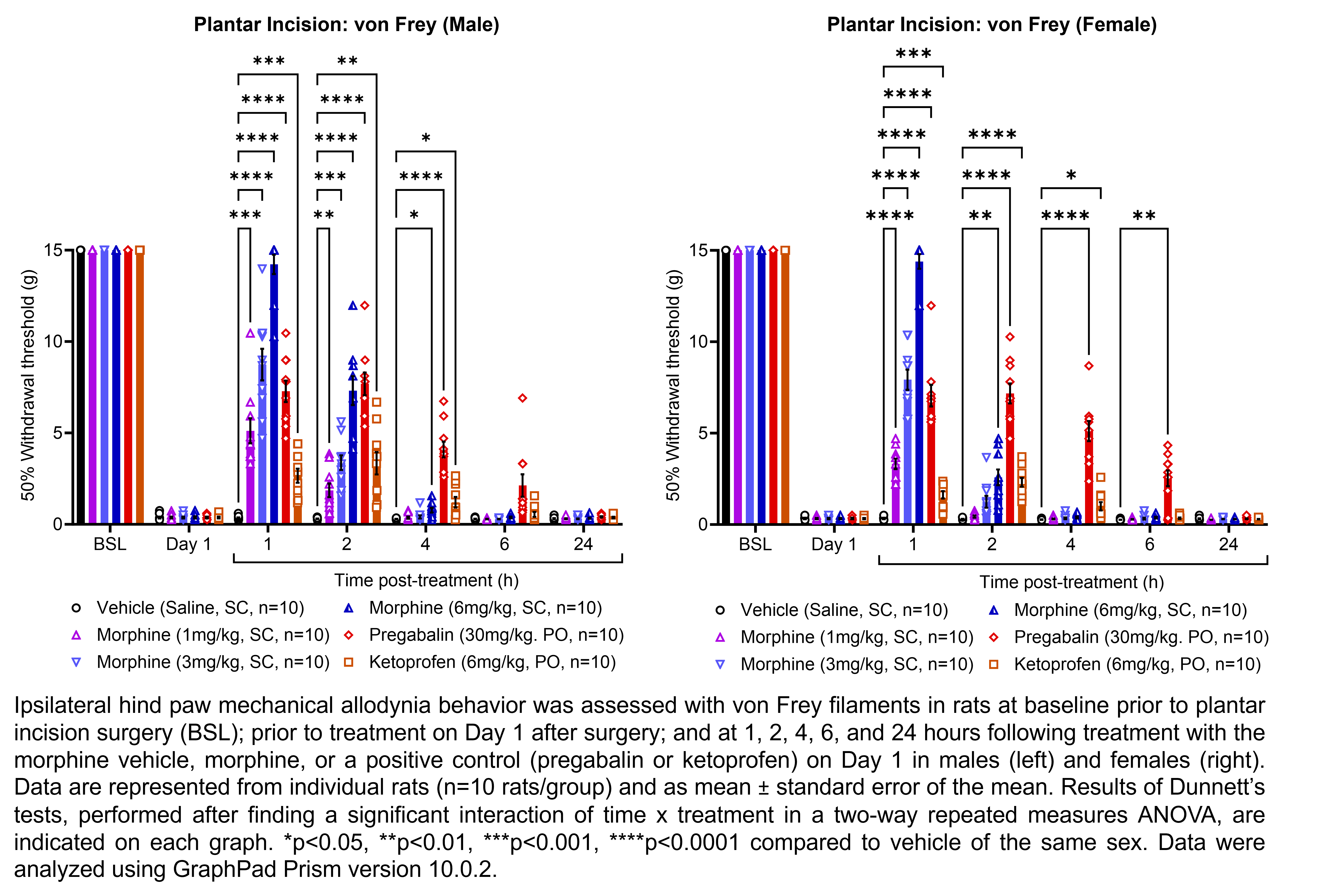
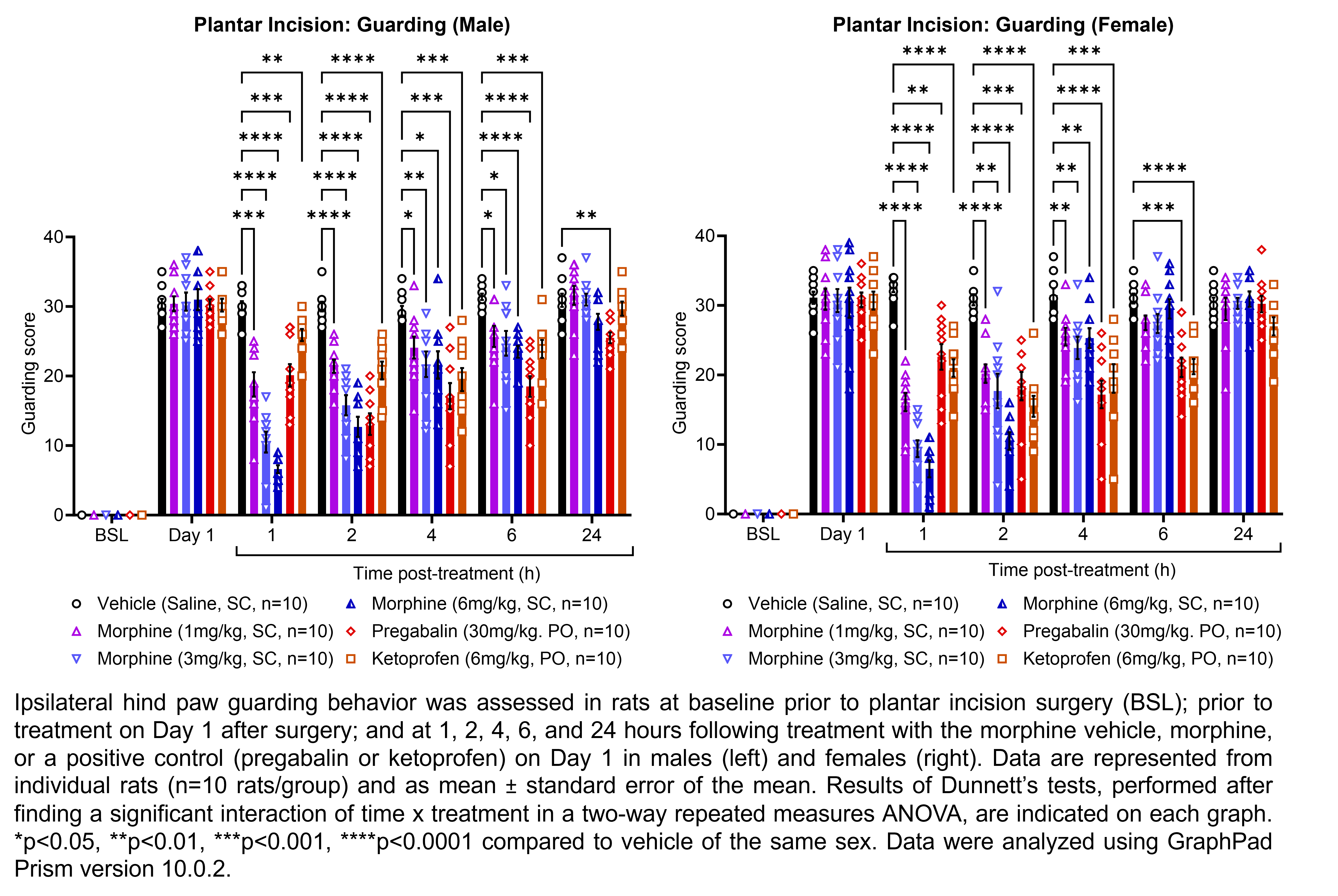
| Plantar incision | Morphine | 1, 3, 6 | 1.33 | 1 | SC | Saline | Solution |
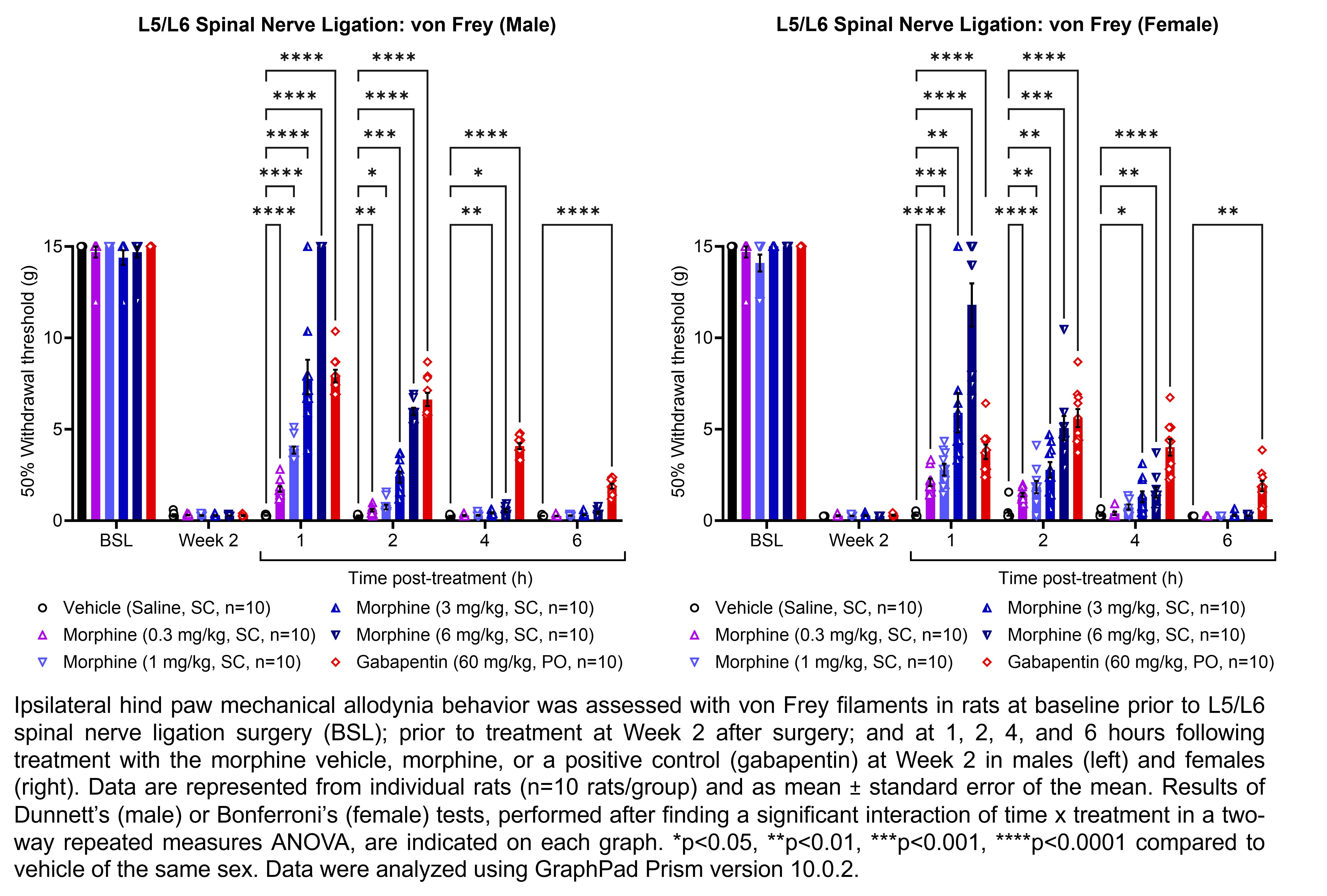
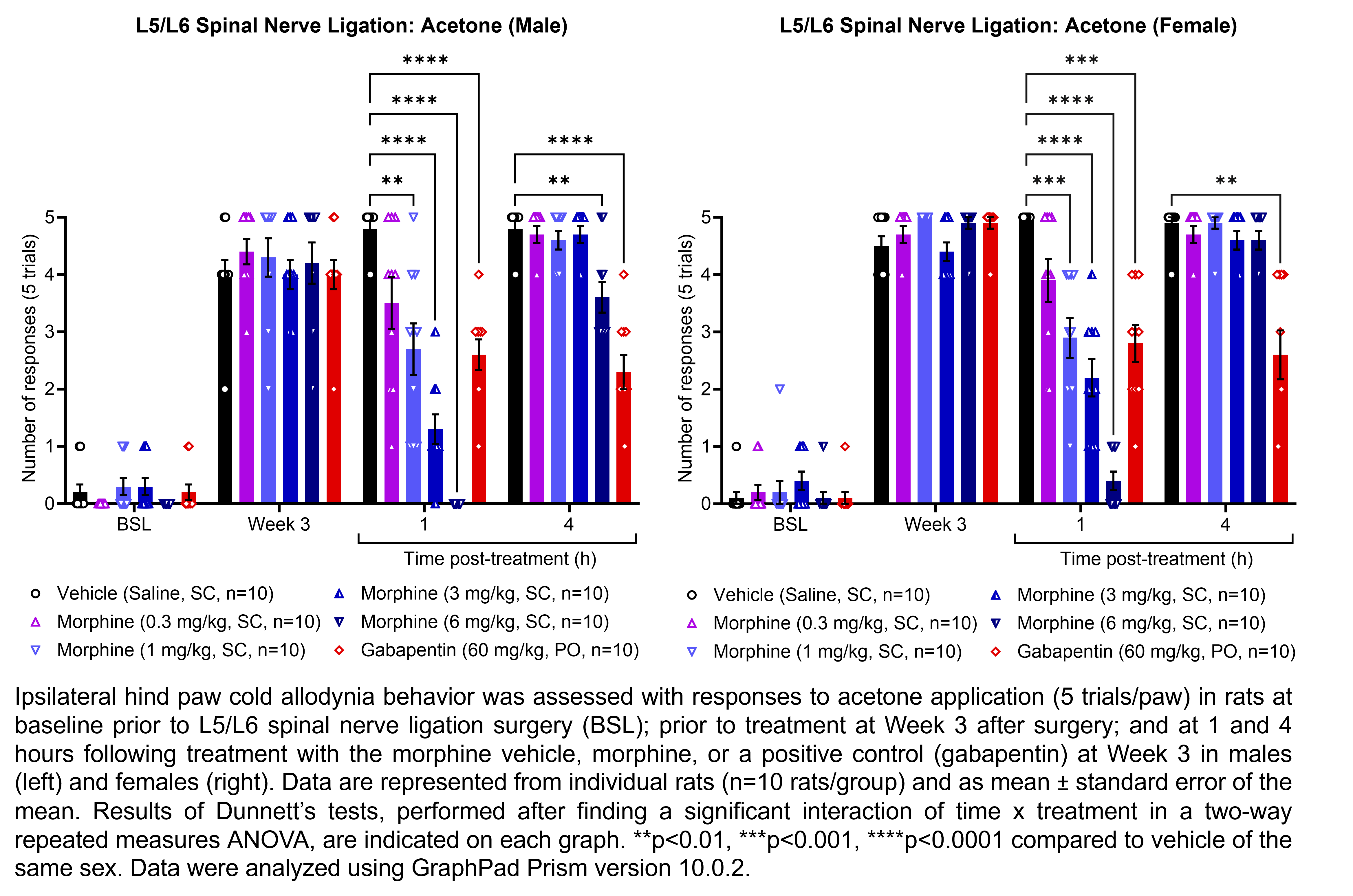
| L5/L6 SNL, MIA, | Morphine | 0.3, 1, 3, 6 | 1.33 | 1 | SC | Saline | Solution |
| Oxaliplatin CIPN | |||||||
| (Acetone) | |||||||
| Paclitaxel CIPN, | Morphine | 0.3, 1, 3 | 1.33 | 1 | SC | Saline | Solution |
| Oxaliplatin CIPN | |||||||
| (von Frey) | |||||||
| Plantar incision | Pregabalin | 30 | 1.00 | 1 | PO | Saline | Solution |
| Plantar incision | Ketoprofen | 6 | 1.00 | 1 | PO | Saline | Suspension |
| L5/L6 SNL | Gabapentin | 60 | 1.00 | 1 | PO | Saline | Solution |
L5/L6 SNL = L5/L6 spinal nerve ligation; MIA = monosodium iodoacetate; CIPN = chemotherapy-induced peripheral neuropathy; PO = per os; SC = subcutaneous
Results
Expand and Collapse accordion content
This work was conducted by PsychoGenics Inc. (Paramus, NJ) in collaboration with PSPP, NINDS, NIH under contract # 75N95019D00026. Prescribing information for clinically used controls can be found at labels.fda.gov. Information for icons representing experimental design details can be found through the NINDS Office of Research Quality https://go.nih.gov/Yw2tHGI.