BUPRENORPHINE
Formulation
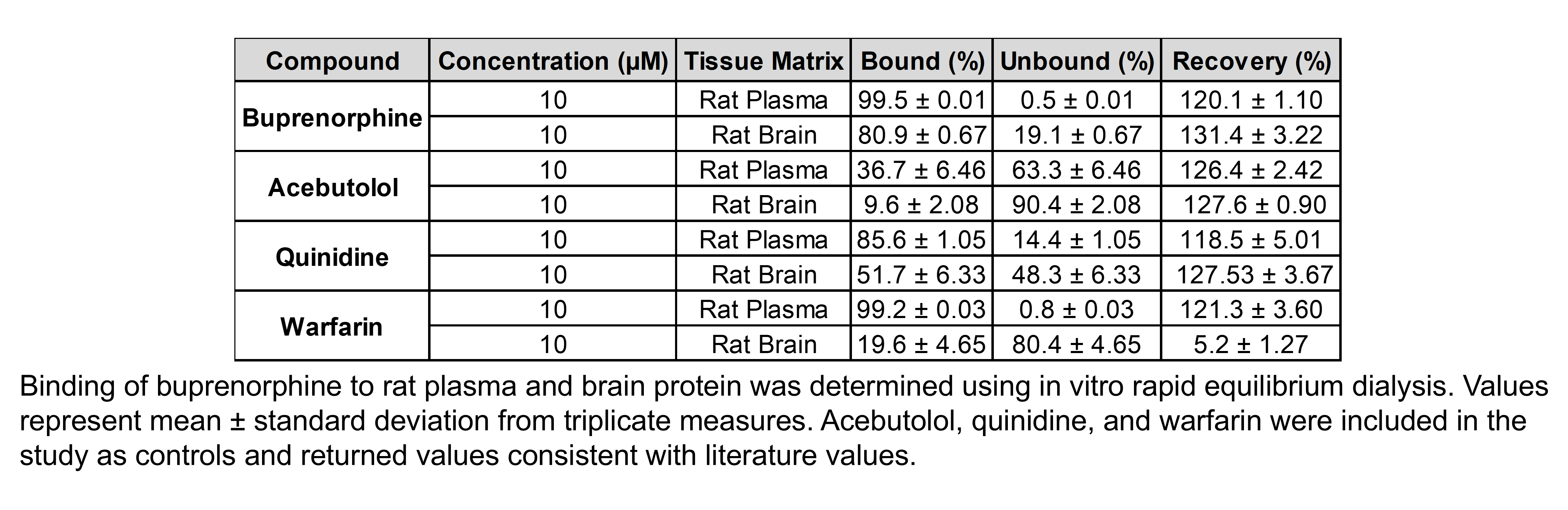
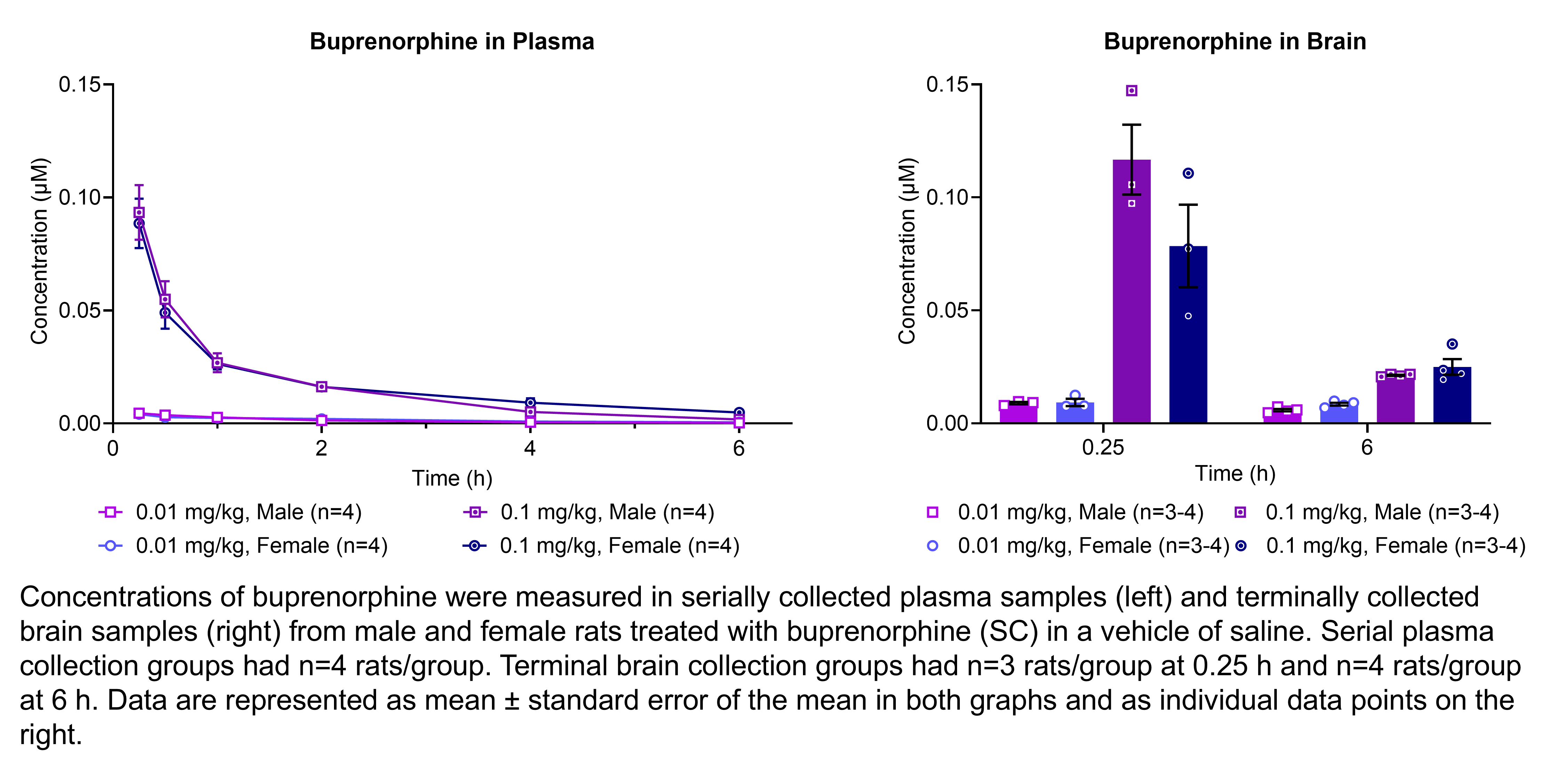
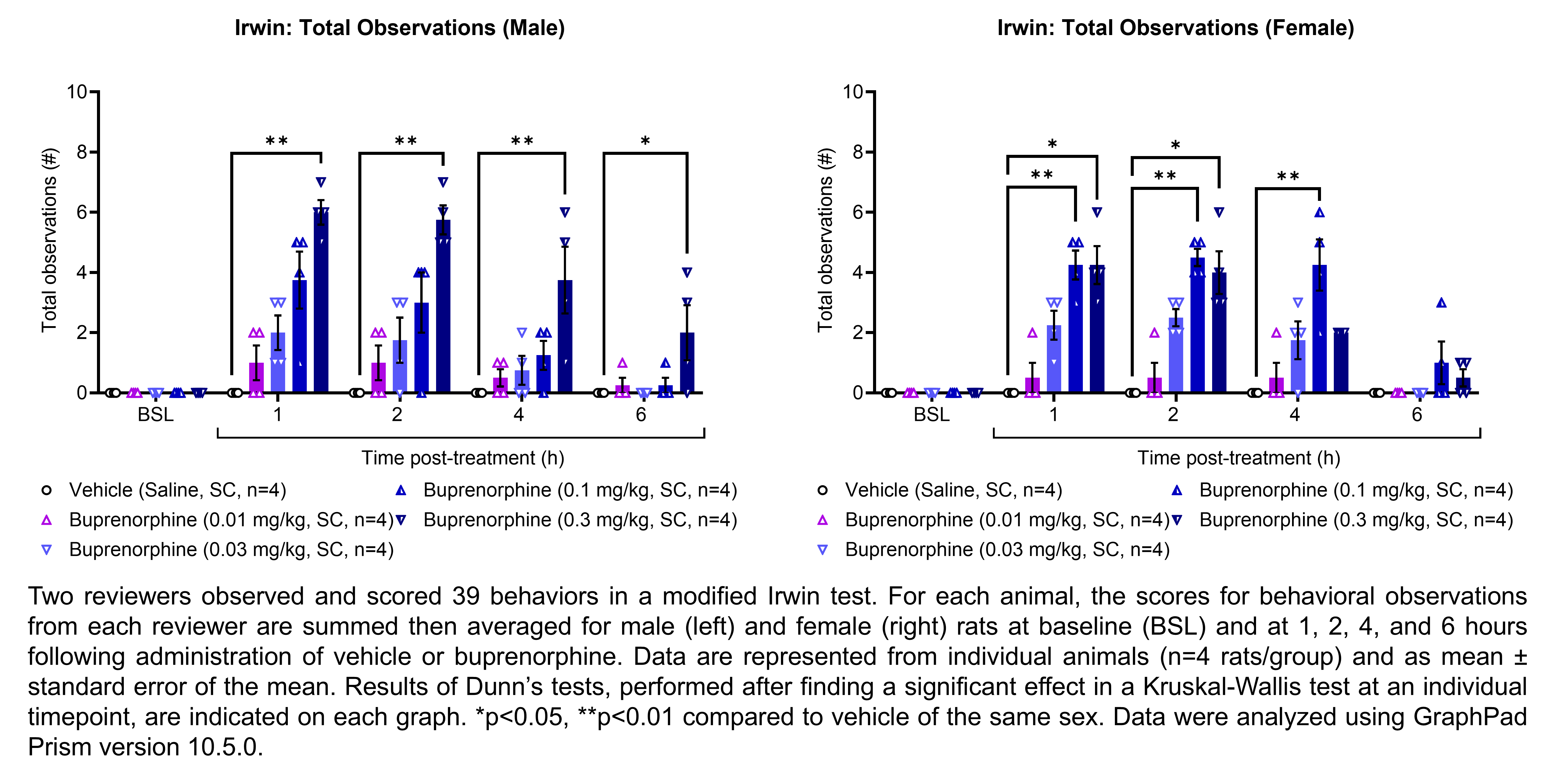
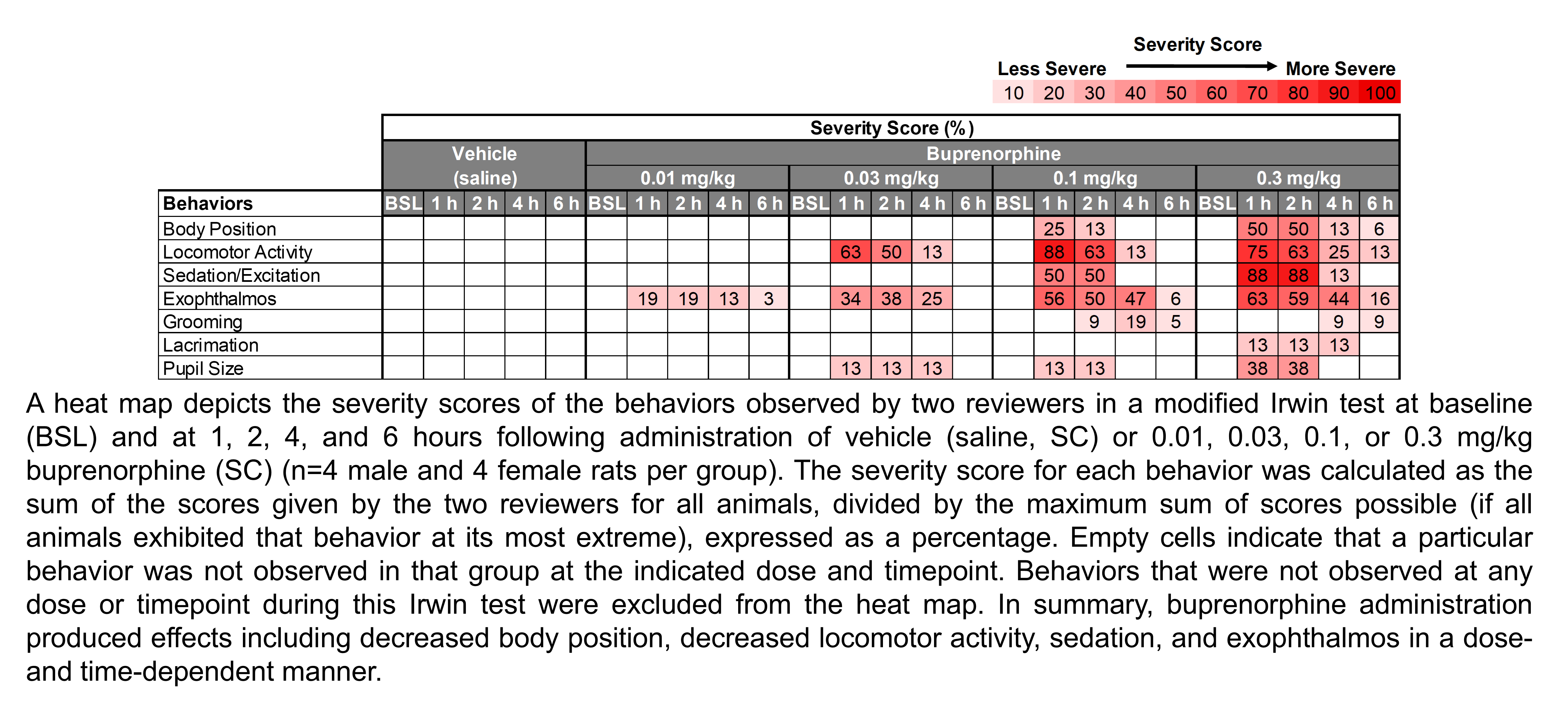
The table below describes the formulation of buprenorphine hydrochloride and the controls morphine sulfate and gabapentin for the following in vivo experiments.
| Experiment(s) | Compound | Dose(s) mg/kg | Correction factor | Dose volume mL/kg | Route | Vehicle | Suspension / Solution |
|---|---|---|---|---|---|---|---|
| Pharmacokinetics | Buprenorphine | 0.01, 0.1 | 1.08 | 1 | SC | Saline | Solution |
| Irwin, Rotarod | Buprenorphine | 0.01, 0.03, 0.1, 0.3 | 1.08 | 1 | SC | Saline | Solution |
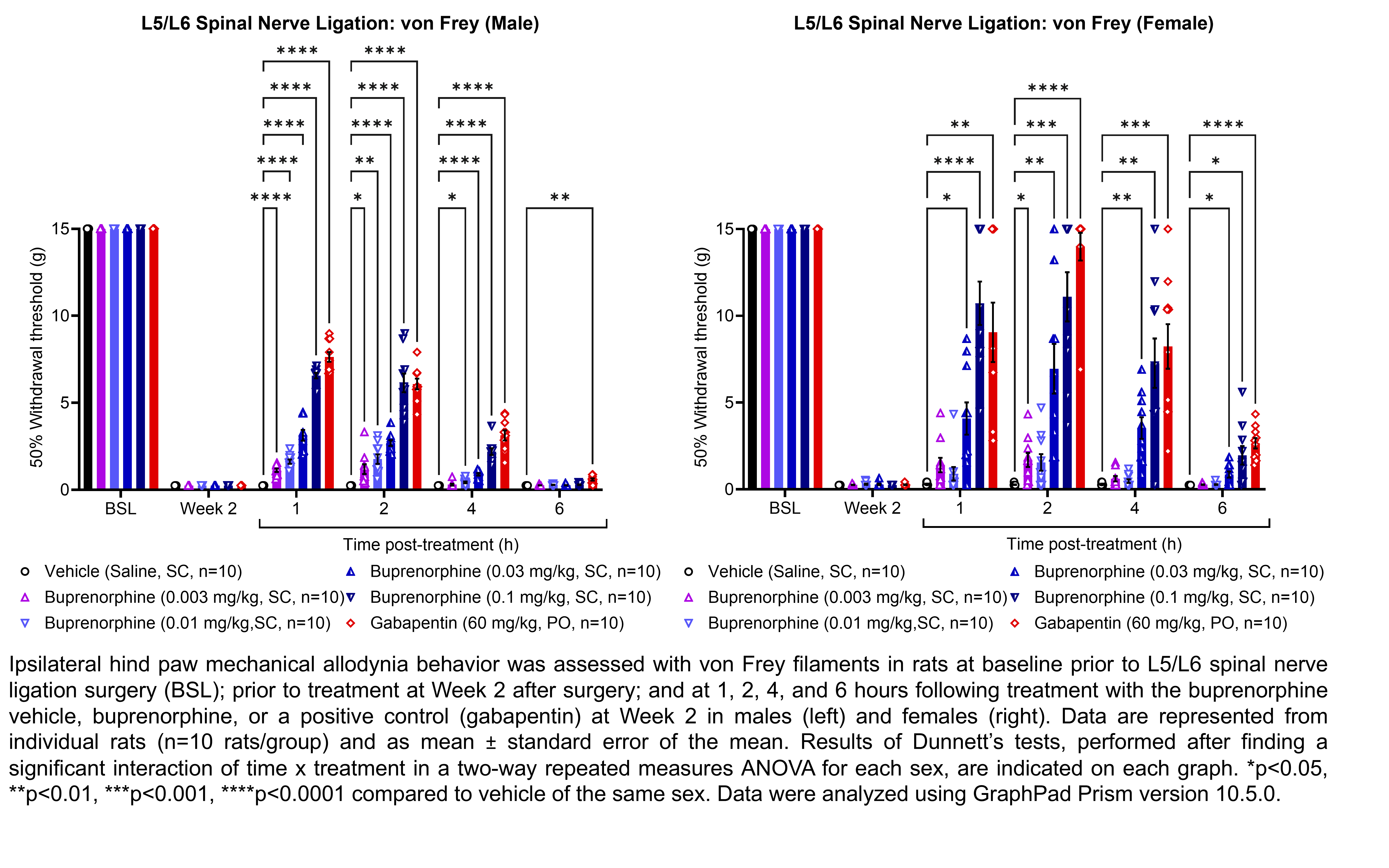
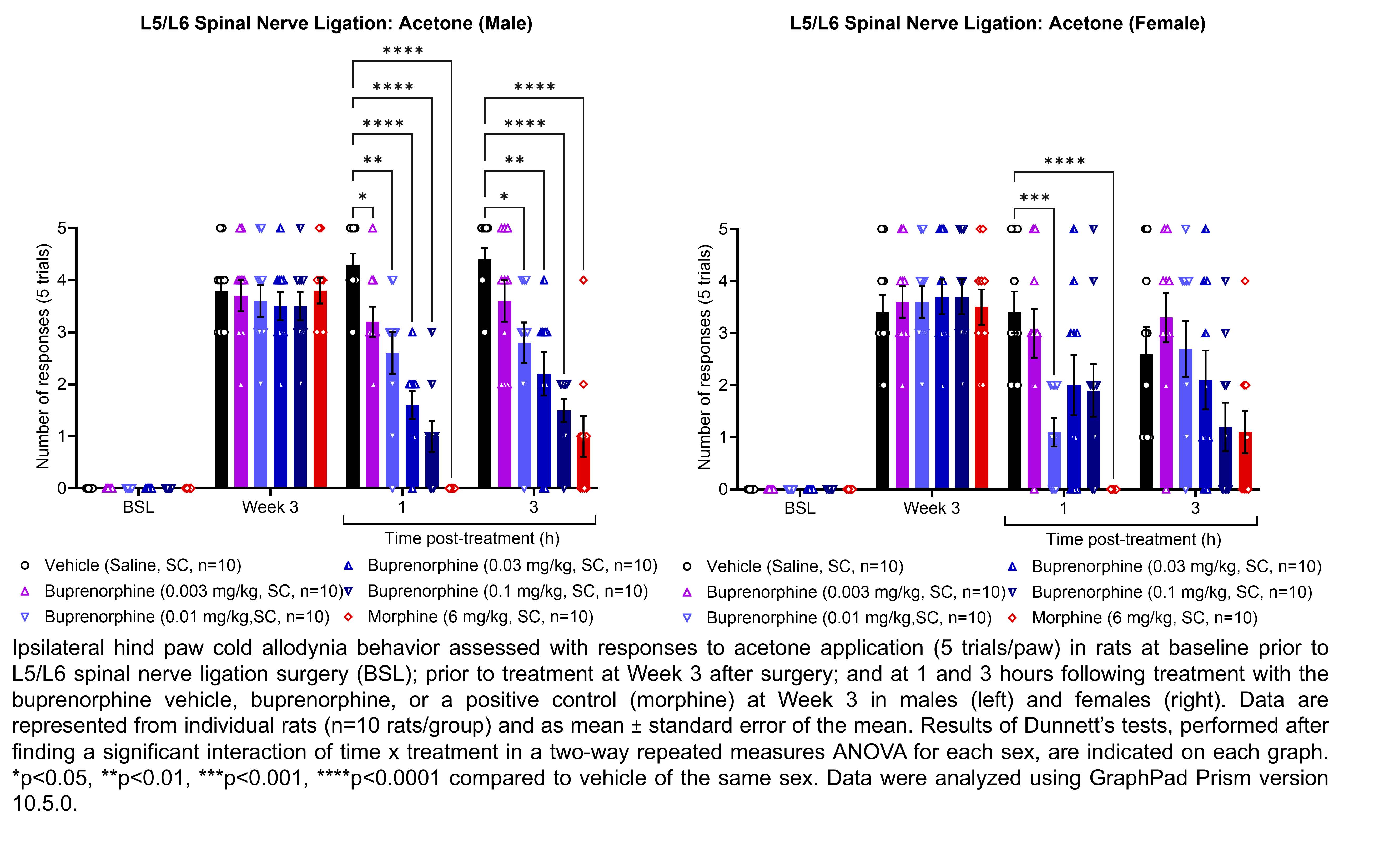
| Plantar incision, | Buprenorphine | 0.003, 0.01, 0.03, 0.1 | 1.08 | 1 | SC | Saline | Solution |
| L5/L6 SNL | |||||||
| Plantar incision | Morphine | 6 | 1.33 | 1 | SC | Saline | Solution |
| L5/L6 SNL | |||||||
| L5/L6 SNL | Gabapentin | 60 | 1.00 | 1 | PO | Saline | Solution |
L5/L6 SNL = L5/L6 spinal nerve ligation; PO = per os; SC = subcutaneous
Results
Expand and Collapse accordion content
This work was conducted by PsychoGenics Inc. (Paramus, NJ) in collaboration with PSPP, NINDS, NIH under contract # 75N95019D00026. Prescribing information for clinically used controls can be found at labels.fda.gov. Information for icons representing experimental design details can be found through the NINDS Office of Research Quality https://go.nih.gov/Yw2tHGI.