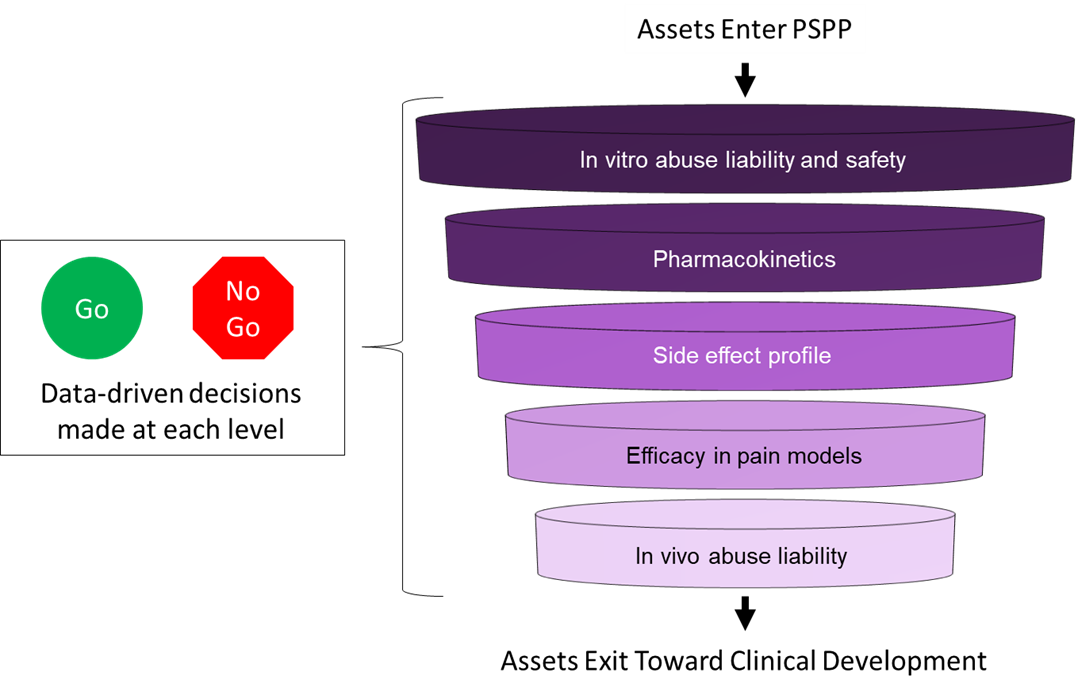
This page describes the workflow that the Preclinical Screening Platform for Pain (PSPP) program uses to evaluate assets accepted into the program. The workflow was determined in collaboration with the PSPP External Consultant Board members and based on outcomes from the Critical Evaluation of Animal Pain Models for Therapeutics Development Workshop in January 2019. As represented in the figure and outline below, the PSPP program uses a stepwise approach to evaluate assets and, after each step of evaluation, data are reviewed before an asset is advanced within the program. Initial asset evaluation focuses on understanding whether there is activity at opioid receptors, activity at known targets associated with abuse liability, or undesirable off-target activity profiles using in vitro functional screens of human receptor preparations. Assets may continue into pharmacokinetic studies in Sprague Dawley male and female rats to understand exposure levels and to select an appropriate dose range and timepoints for the subsequent behavioral assessments. Assets that advance to the next step are evaluated in a set of validated assays to identify any potential neurological side effects. The data from all evaluations are then used to set a dose range for the evaluation of efficacy in validated preclinical pain models, including an acute to sub-chronic model (plantar incision) and a chronic/persistent model (L5/L6 spinal nerve ligation). To further identify the specific pain profile for an asset, the efficacy can also be assessed further in disease-specific pain models, such as paclitaxel-induced peripheral neuropathy. For each pain model, one or more validated endpoints are used to evaluate potential analgesic efficacy of the asset. Finally, assets with demonstrated efficacy can be evaluated for abuse liability in vivo. Detailed information about each assay, including descriptions of the validated pain models and endpoints, are linked below. Importantly, additional models and endpoints will be evaluated continuously for possible incorporation into the PSPP program.